Mylab Launches First Made in India Kit and Automated Devices for TB and Multi-drug Resistance
Home » Mylab Launches First Made in India Kit and Automated Devices for TB and Multi-drug Resistance
30 Nov 2022 | Pune
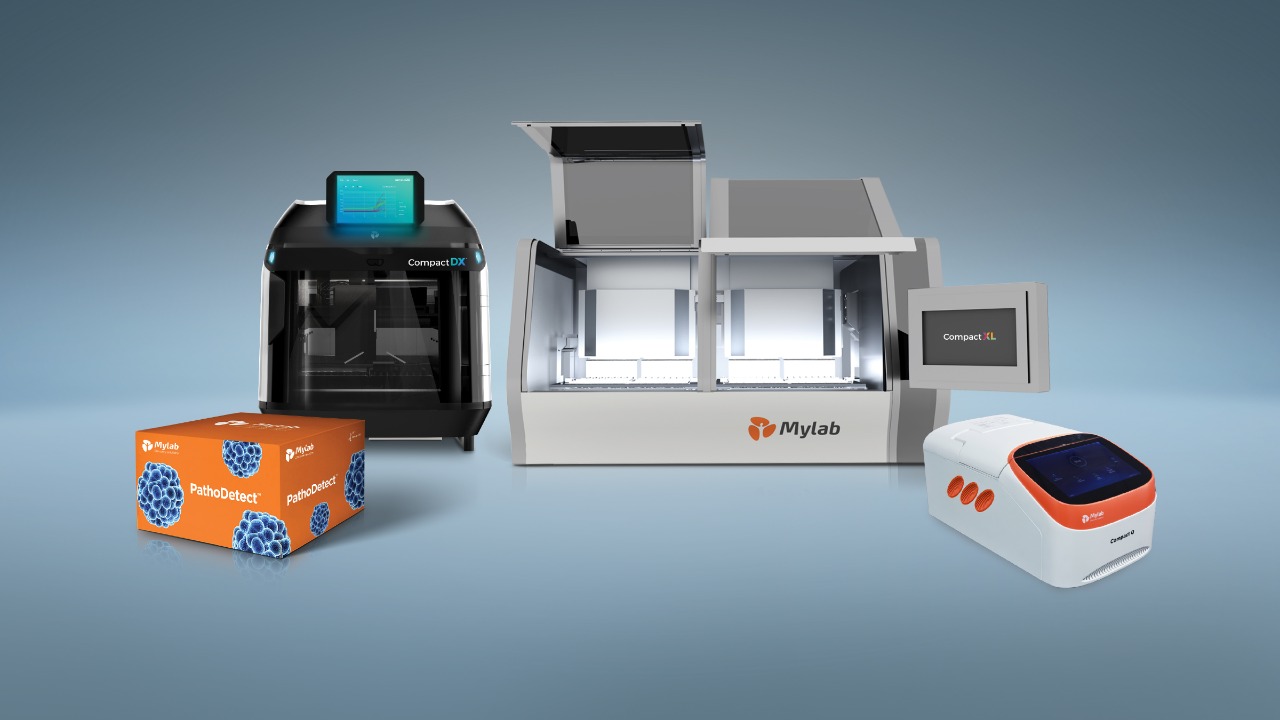
Mylab, after leading the diagnostics innovations for Covid-19, has received CDSCO, TB Expert Committee and ICMR approval for first Made in India TB Detection kit to detect Tuberculosis which simultaneously detects multiple rug resistance to Rifampicin and Isoniazid in single test. The kit is named PathoDetect™ MTB RIF and INH drug resistance kit. The kit is a RT-PCR based kit for accurate detection and will be used with Mylab Compact™ device systems – which will allow completely automated testing of multiple samples within 2 hours. This will be instrumental in supporting the honourable Prime minister’s vision to eliminate TB by 2025 from India.
This PathoDetect™ kit combined with Mylab Compact™ device platform will fill the current gaps in Tuberculosis testing, a disease which caused more deaths every year than the Covid-19 second wave. Explaining how this kit gives better testing option for TB, Hasmukh Rawal, the MD of Mylab said, “We are addressing several problems simultaneously here. First being able to speed up testing by automated systems that can do multiple tests at one time. Secondly, there is scarce trained manpower for RT-PCR testing, which India can now overcome with fully automated system which does not need highly technical person to handle samples and reagents.”
The kit has been approved after rigorous and large scale field trials and recommended by TB Expert Committee under the aegis of ICMR. Multicentre centre evaluation study and field feasibility testing studies were carried out for the “PathoDetect MTB RIF and INH drug resistance kits” & Compact device systems. The centres of trials included the most reputed Tb research centres of India, which evaluated the performance of “PathoDetect™ MTB RIF and INH drug resistance kit“ against the currently used diagnostic assays for Tuberculosis.
Emphasizing on the important point of drug resistance, Hasmukh Rawal said, “There is a huge problem of resistance to drugs when it comes to TB. Until now, India had to conduct 2 tests: one to detect TB first and to check drug resistance – that against only one drug (Rifampicin). With Mylab’s PathoDetect™ kit, in a single test – patients can know their active TB infection as well as drug resistance to 2 most common drugs – Isoniazid and Rifampacin – so that they take treatment that will actually work. This is a milestone moment in India’s TB Testing.”
With automation, the complete testing process even at rural areas and mobile labs. The test kits have been designed to work in ambient temperatures compared to existing PCR options which need 2-8 degree cold storage. Mylab Compact™ device systems do not require special infrastructure for operations and feasibility studies done on Mobile Van in rural areas indicate them to be very robust. The kits as well as device are fully indigenous ‘Make in India’ product.
Appendix
The centres involved in this evaluation study included
Mylab, after leading the diagnostics innovations for Covid-19, has received CDSCO, TB Expert Committee and ICMR approval for first Made in India TB Detection kit to detect Tuberculosis which simultaneously detects multiple rug resistance to Rifampicin and Isoniazid in single test. The kit is named PathoDetect™ MTB RIF and INH drug resistance kit. The kit is a RT-PCR based kit for accurate detection and will be used with Mylab Compact™ device systems – which will allow completely automated testing of multiple samples within 2 hours. This will be instrumental in supporting the honourable Prime minister’s vision to eliminate TB by 2025 from India.
This PathoDetect™ kit combined with Mylab Compact™ device platform will fill the current gaps in Tuberculosis testing, a disease which caused more deaths every year than the Covid-19 second wave. Explaining how this kit gives better testing option for TB, Hasmukh Rawal, the MD of Mylab said, “We are addressing several problems simultaneously here. First being able to speed up testing by automated systems that can do multiple tests at one time. Secondly, there is scarce trained manpower for RT-PCR testing, which India can now overcome with fully automated system which does not need highly technical person to handle samples and reagents.”
The kit has been approved after rigorous and large scale field trials and recommended by TB Expert Committee under the aegis of ICMR. Multicentre centre evaluation study and field feasibility testing studies were carried out for the “PathoDetect MTB RIF and INH drug resistance kits” & Compact device systems. The centres of trials included the most reputed Tb research centres of India, which evaluated the performance of “PathoDetect™ MTB RIF and INH drug resistance kit“ against the currently used diagnostic assays for Tuberculosis.
Emphasizing on the important point of drug resistance, Hasmukh Rawal said, “There is a huge problem of resistance to drugs when it comes to TB. Until now, India had to conduct 2 tests: one to detect TB first and to check drug resistance – that against only one drug (Rifampicin). With Mylab’s PathoDetect™ kit, in a single test – patients can know their active TB infection as well as drug resistance to 2 most common drugs – Isoniazid and Rifampacin – so that they take treatment that will actually work. This is a milestone moment in India’s TB Testing.”
With automation, the complete testing process even at rural areas and mobile labs. The test kits have been designed to work in ambient temperatures compared to existing PCR options which need 2-8 degree cold storage. Mylab Compact™ device systems do not require special infrastructure for operations and feasibility studies done on Mobile Van in rural areas indicate them to be very robust. The kits as well as device are fully indigenous ‘Make in India’ product.
Appendix
The centres involved in this evaluation study included
- National Jalma Institute of Leprosy and other Mycobacterial Diseases(JALMA), Agra
- National Institute of Tuberculosis and Respiratory Diseases (NITRD), New Delhi
- National Institute for Research in Tuberculosis (NIRT), Chennai
- National Institute of Research in Tribal Health (NIRTH), Jabalpur
- Regional Medical Research Center (RMRC), Bhubaneswar
- Bhopal Memorial Hospital and Research Centre (BHMRC), Bhopal
For media queries, reach out to pr@mylabglobal.com
For sales queries, reach out to info@mylabglobal.com
For sales queries, reach out to info@mylabglobal.com